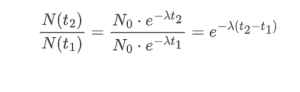In nuclear physics, the term “half-life” refers to the time it takes for half of a quantity of a radioactive substance to undergo decay. If you are a student of archaeology or medicine then you need to get yourself familiarized with this concept at all cost. Our Half-Life Calculator is here to help you with that.
We will guide you through understanding and computing half-lives with ease, employing clear explanations and practical tools. Discover how a half-life calculator simplifies this scientific task!
Understanding Half Life
Diving into the heart of nuclear physics, we uncover the concept of half-life, a pivotal tool for understanding the rate at which radioactive substances decay. First identified by Ernest Rutherford, this measure reveals the ephemeral nature of even the most seemingly indestructible elements.
Definition And Concept
Half-life is a way to measure how long it takes for half of something that is decaying to go away. It’s like a clock for stuff that breaks down slowly over time, such as certain elements. Here is the formula:
Imagine you have a big pile of radioactive atoms. In science talk, these atoms are “unstable,” which means they can’t stay the same forever and will eventually change into different atoms by releasing some energy or tiny bits called particles.
Ernest Rutherford found out about half-life in 1907. He saw that unstable stuff didn’t all change at once but took its own sweet time, losing half its amount bit by bit. The cool thing is, no matter how much of this unstable stuff you have – be it little or lots – exactly half of it will decay after each period known as the “half-life.” This happens in patterns we can predict with math and science magic!
Discovery By Rutherford
In 1907, Rutherford made a big find. He found out about half-life and changed how we see radioactive decay. His work helped us understand why some things break down over time. Thanks to him, we got new ways to learn about old bones and rocks through carbon dating.
Rutherford’s breakthrough was huge for science. It started more studies on how atoms change and fall apart. Because of what he did, we now know a lot about nuclear physics and can do many cool things with that knowledge.
Half-Life Calculation
Understanding how to calculate half-life is crucial in fields ranging from archaeology with radiocarbon dating to medicine with pharmacokinetics. This process involves applying the decay constant in an exponential formula that reveals the time it takes for a substance to reduce to half its initial quantity.
Let’s delve into the mathematical journey of determining this pivotal parameter, empowering you with the ability to quantify radioactive decay and its implications across various applications.
Using Radioactive Decay Formula
To use the radioactive decay formula, you first need to know a few things. These include the amount of time that has passed and how much of the substance you started with. Then there’s this special number called ‘decay constant’ (λ) which is important for figuring out how fast something decays.
Imagine you’re looking at a piece of wood from an old shipwreck and want to know its age. Scientists can measure it using carbon-14 dating. They do some math with their half-life formula like T = t_{1/2} ln(N_t/N_0)/–ln(2).
This helps them find out when that ship might have sailed! It’s kind of like having a time machine in numbers and equations, getting clues from what remains today to tell stories about the past.
Steps To Calculate Half-Life
Calculating half-life helps us understand how fast stuff breaks down. It’s like a stopwatch for atoms that fall apart. Here’s how you do it:
- Find out how much of the element you start with. This is your initial quantity.
- Work out how much is left over after some time. This is your remaining quantity.
- Measure the time it took for the material to break down from the starting amount to what’s left.
- Grab your calculator and use this formula: T = (t_1/2 * ln(N_t/N_0)) / –ln(2), where “T” is the half-life, “t_1/2” is the time taken, “N_t” is the remaining quantity, and “N_0” is the initial quantity.
- Type in what you started with and what’s left into a decay constant calculator.
- The calculator will tell you the half-life based on what you typed in.
Types of Radioactive Decay
Understanding the types of radioactive decay is pivotal in grasping how elements transform over time. This segment delves into the mechanisms behind alpha, beta, and gamma decay, each characterized by distinct particle emissions and energy releases that alter an atom’s core identity.
1. Alpha Decay
Alpha decay makes big atoms smaller. Imagine a really heavy atom, like uranium-233. It’s got too many protons and neutrons to hold together well. This atom wants to be stable, so it kicks out a tiny clump made of 2 protons and 2 neutrons – that’s an alpha particle! When the alpha particle flies away, it takes some mass with it. What’s left is now a new element that’s lighter and more stable.
Think of this as radioactive elements trying to find balance. Big elements break down over time into something less massive by releasing energy and bits like the helium nucleus – an alpha particle.
Watch for these changes when you study unstable nuclei; they show us how nature seeks stability through alpha decay.
2. Beta Decay
Just like alpha decay changes an atom by sending out alpha particles, beta decay works a bit differently. In beta decay, an unstable atom loses energy by shooting out a particle that’s either an electron or a positron. This happens when a neutron in the atom turns into a proton or the other way around.
Think of it like this: inside the atomic nucleus, there’s kind of a team switch. A player from Team Neutron jumps to Team Proton (or the opposite), and to keep things balanced, they toss out a tiny beta particle.
This is important because it can change one element into another. For example, carbon-14, which archaeologists use to tell how old things are, decays over time by losing these beta particles.
Beta particles are fast and can be stopped by just a few sheets of paper or even your skin! But you still have to be careful with them because they’re part of why some substances are radioactive.
Even though we don’t cover all the details about beta decay here, knowing what it is helps us understand how elements transform and why some materials can be dangerous due to radioactivity.
3. Gamma Decay
Gamma decay happens when an atom’s nucleus releases energy. This kind of decay doesn’t change the number of protons or neutrons in the nucleus, but it does make the nucleus drop to a lower energy state.
When this happens, gamma rays come out. Gamma rays are a type of light that has more energy than visible light.
Understanding gamma decay is important for learning about unstable nuclei in heavier elements. As they let go of excess energy as gamma rays, these atoms move toward being stable. It’s like shaking off extra weight to feel better.
Using this knowledge helps us figure out how and why atoms give off different kinds of radiation, which is key in fields like medicine and industry where we use radioactive materials safely and helpfully.
Examples of Half-Life
From the fascinating age determination using Carbon-14 to the medical applications of Thorium, understanding examples of half-life reveals a world where time is encoded in atoms—continue reading to uncover these atomic chronicles.
Carbon 14
Carbon 14 is a type of carbon that scientists pay close attention to. It’s different from regular carbon because it has two extra neutrons, making it unstable. Over time, it changes into a different element by shooting out particles from its nucleus—this process is called radioactive decay.
People use Carbon 14 to date old things like bones and trees because we know how long its half-life is.
Knowing the half-life of Carbon 14 helps us understand how old some things are. The half-life of this special carbon is about 5730 years. That means after 5730 years, only half of the Carbon 14 in something will be left; the rest turns into another element.
This clock-like nature makes it really handy for figuring out when ancient living things died and became part of history!
Other Elements
Just like Carbon 14, other elements also have half-lives that play a key role in different fields. These elements decay over time, releasing particles and changing into other elements or isotopes.
- Uranium-238: It decays slowly with a half-life of about 4.5 billion years. Scientists use it to date rocks and the age of Earth.
- Radon gas: With a half-life of 3.8 days, radon is important in studying earthquakes and monitoring the environment.
- Iodine-131: It has a short half-life of 8 days. Doctors use it to treat thyroid problems.
- Strontium-90: Its half-life is around 29 years. This element can be harmful because it may stay in bones for a long time.
- Plutonium-239: This element’s half-life is 24,100 years. People use it as fuel in nuclear reactors and to make nuclear weapons.
- Technetium-99m: With just 6 hours of half-life, this is very useful in medical scans to see inside the body.
Benefits of Half-Life Calculator
Harnessing a Half-Life Calculator streamlines the complex process of determining decay rates, delivering precise and swift results that enhance both understanding and efficiency in scientific exploration—dive deeper to discover its pivotal role in modern calculations.
1. Time-Saving Automation
Calculating half-life by hand takes a lot of steps and can be tricky. However, using a half-life calculator makes it much faster and easier. You type in the numbers, and it does all the hard math for you.
It’s like having a smart helper that always gets the math right. This tool helps you quickly find out how much of something is left after time passes.
Now, let’s talk about how dependable these results are.
2. Reliable And Accurate Results
Half-life calculators are truly helpful tools. They give you the exact numbers you need to understand how much of a radioactive substance is left over time. You can trust these results because they use well-tested formulas.
For example, when scientists say that carbon 14 takes about 5730 years to reduce by half, they’re confident in this number.
These calculators save you from doing tough math on your own. Just type in what you know—the starting amount and the decay constant—and the calculator works out the rest fast. This means less chance for mistakes and more trust in your answers, whether it’s for school work or advanced science projects.
FAQs
Question: What Is Half-Life In Simple Terms?
Half-life is the time it takes for half of something that falls apart, like a radioactive atom, to break down into something else. It’s like a countdown for unstable things turning stable.
Question: How Do You Use A Calculator To Find The Half-Life?
To use a calculator for finding half-life, you need two main bits of info: how much stuff you started with and how much is left after some time has passed. Plug these numbers along with the decay constant into the calculator, and it tells you the half-life.
Question: What Does Decay Constant Mean?
The decay constant is a number that shows how fast an unstable nucleus in stuff like uranium-232 breaks down into smaller parts. This number helps figure out when exactly things will fall apart or change inside what’s being looked at.
Question: Can We Calculate The Exact Time When All Radioactive Material Will Be Gone?
Nope! We can’t tell exactly when all radioactive material will disappear because it decays exponentially over time. But using mean lifetime and other parameters, we can guess when most of it should be gone.
Question: Why Do Scientists Care About Carbon Dating?
Scientists dig carbon dating because it allows them to figure out how old things are by measuring the decay of carbon-14 inside them compared to stable nuclides like carbon-12.
Question: Does Anything Else Come Out Besides Smaller Atoms During Nuclear Decay?
Oh yes! When stuff breaks down during nuclear decay, little particles fly off too—like electrons (beta particles), neutrinos from electron capture, positrons if there’s internal conversion happening, or gamma emissions from photons escaping.
Conclusion
Understanding half-life and how to calculate it is key for many science tasks. The concept of half-life is crucial in understanding the rate of radioactive decay and is widely used in various fields such as archaeology, geology, medicine, and environmental science.
You can learn a lot by using the right tools and methods we talked about. Remember, mastering these steps can open new doors in your learning journey! If you have any questions or problems, let us know in the comments below!